Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Arai, Shigeki; Adachi, Motoyasu; Kawamoto, Masahide*; Tokunaga, Hiroko*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
no journal, ,
We attempted to discover the Cs and Sr
and Sr binding sites on the halophilic
binding sites on the halophilic  -Lactamase (HaBLA) derived from
-Lactamase (HaBLA) derived from  sp.560 by the anomalous X-ray diffraction analysis. One Cs
sp.560 by the anomalous X-ray diffraction analysis. One Cs ions in the HaBLA crystal were identified by BL7 at SAGA-LS. Three Sr
ions in the HaBLA crystal were identified by BL7 at SAGA-LS. Three Sr ions in the HaBLA crystal were identified by BL38B1 at SPring-8 and NW12A at Photon Factory. Discovered Cs
ions in the HaBLA crystal were identified by BL38B1 at SPring-8 and NW12A at Photon Factory. Discovered Cs binding site can bind Cs
binding site can bind Cs selectively from a solution containing Na
selectively from a solution containing Na /Cs
/Cs = 90 mM/10 mM. This Cs
= 90 mM/10 mM. This Cs binding site is formed by two main-chain O atoms and an aromatic ring of a side chain of Trp. An aromatic ring of Trp interacts with Cs
binding site is formed by two main-chain O atoms and an aromatic ring of a side chain of Trp. An aromatic ring of Trp interacts with Cs by the cation-
by the cation- interaction. The observation of metal binding sites with relatively higher Cs
interaction. The observation of metal binding sites with relatively higher Cs selectivity provides important information that is useful for designing artificial Cs
selectivity provides important information that is useful for designing artificial Cs binding sites.
binding sites.
 from porcine liver
from porcine liverHirano, Yu; Kimura, Shigenobu*; Tamada, Taro
no journal, ,
Mammalian microsomal cytochrome  (b5) is a hemoprotein which anchored to the membrane of the endoplasmic reticulum and it is involved in various electron transfer reactions, such as lipid unsaturation, cholesterol synthesis and drug metabolism. The 1.5
(b5) is a hemoprotein which anchored to the membrane of the endoplasmic reticulum and it is involved in various electron transfer reactions, such as lipid unsaturation, cholesterol synthesis and drug metabolism. The 1.5  , structure of b5 from bovine liver reveals that acidic residues located in the vicinity of heme play a role in interaction with electron transfer partners. To further understand the electron transfer mechanism based on the precious structural information, we attempted the structure determination above 1
, structure of b5 from bovine liver reveals that acidic residues located in the vicinity of heme play a role in interaction with electron transfer partners. To further understand the electron transfer mechanism based on the precious structural information, we attempted the structure determination above 1  , resolution of both oxidized (OX) and reduced (RD) form of porcine liver b5. We obtained four types of b5 crystals, OX with/without calcium ion (Ca
, resolution of both oxidized (OX) and reduced (RD) form of porcine liver b5. We obtained four types of b5 crystals, OX with/without calcium ion (Ca ) and RD with/without Ca
) and RD with/without Ca , and then collected each dataset above 1
, and then collected each dataset above 1  , resolution, 0.85
, resolution, 0.85  , (OX + Ca
, (OX + Ca ), 0.93
), 0.93  ,(OX - Ca
,(OX - Ca ), 0.85
), 0.85  , (RD + Ca
, (RD + Ca ), and 0.95
), and 0.95  , (RD - Ca
, (RD - Ca ). In the both structures of OX and RD form with Ca
). In the both structures of OX and RD form with Ca , two glutamate located in the vicinity of heme participated in the recognition of Ca
, two glutamate located in the vicinity of heme participated in the recognition of Ca . From the comparison among four structures, we confirmed that the structural difference between with and without Ca
. From the comparison among four structures, we confirmed that the structural difference between with and without Ca (RMSD: 0.61-0.65
(RMSD: 0.61-0.65  ) is larger than that between OX and RD form (RMSD: 0.12-0.23
) is larger than that between OX and RD form (RMSD: 0.12-0.23  ).
).
Kono, Hidetoshi; Ikebe, Kimiyoshi
no journal, ,
no abstracts in English
Shibazaki, Chie; Adachi, Motoyasu; Shimizu, Rumi; Kuroki, Ryota
no journal, ,
Casein kinase 2(CK2) is one of the ubiquitous Ser/Thr kinases and is involved in the cell cycle and the survival and proliferation of cells. CK2 is a heterotetrameric structure comprising two  - or
- or  -subunits. In order to understand the biological function of the alpha catalytic subunit of CK2 (CK2
-subunits. In order to understand the biological function of the alpha catalytic subunit of CK2 (CK2 ), we aim to analyze the structure of CK2
), we aim to analyze the structure of CK2 including information of the hydrogen and hydrating water molecule by neutron crystallography. The gene coding CK2
including information of the hydrogen and hydrating water molecule by neutron crystallography. The gene coding CK2 was inserted into pET24a and expressed in
was inserted into pET24a and expressed in 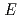 . coli strain BL21DE3, in which the mobile region (330-335) and chemically reactive thiols (Cys147 and Cys220) were removed by amino acid mutation. A total of 150 mg protein was obtained from a 6 L culture, and was used for crystallization trials. The preparation of large crystals was performed using a macro seeding method. Finally, a large crystal with a volume of approximately 2 mm
. coli strain BL21DE3, in which the mobile region (330-335) and chemically reactive thiols (Cys147 and Cys220) were removed by amino acid mutation. A total of 150 mg protein was obtained from a 6 L culture, and was used for crystallization trials. The preparation of large crystals was performed using a macro seeding method. Finally, a large crystal with a volume of approximately 2 mm was reproducibly obtained. The crystal was dialyzed in the deuterated reagent and deuterium water, and the preliminary neutron diffraction experiments were carried out at neutron beam line BioDIFF in research reactor FRM-II in Technical University of Munich. The diffraction was successfully observed greater than 1.9
was reproducibly obtained. The crystal was dialyzed in the deuterated reagent and deuterium water, and the preliminary neutron diffraction experiments were carried out at neutron beam line BioDIFF in research reactor FRM-II in Technical University of Munich. The diffraction was successfully observed greater than 1.9  resolution.
resolution.
Hiromoto, Takeshi; Adachi, Motoyasu; Shibazaki, Chie; Kuroki, Ryota
no journal, ,
T4 phage lysozyme (T4L) is an endoacetylmuramidase that degrades the murein of the bacterial cell wall by cleavage of the  -1,4-glycosidic bond between
-1,4-glycosidic bond between  -acetylmuramic acid and
-acetylmuramic acid and  -acetylglucosamine. We previously reported that the substitution of the catalytic Thr26 to the nucleophilic His converts the wild type (WT) T4L from an inverting to a retaining glycosidase, in which the
-acetylglucosamine. We previously reported that the substitution of the catalytic Thr26 to the nucleophilic His converts the wild type (WT) T4L from an inverting to a retaining glycosidase, in which the  -configuration of the substrate is retained in the product. It was also found that the Thr26His mutant T4L can catalyze the transglycosylation reaction more effectively than hydrolysis although the WT T4L has no transglycosidase activity. To clarify the role of the substituted His26 on transglycosylation and its relationship to the neighboring acidic residue Asp20 by neutron crystallography, the perdeuterated recombinant proteins of the WT and Thr26His mutant T4L were prepared for crystallization in this study. The perdeuterated forms were produced in
-configuration of the substrate is retained in the product. It was also found that the Thr26His mutant T4L can catalyze the transglycosylation reaction more effectively than hydrolysis although the WT T4L has no transglycosidase activity. To clarify the role of the substituted His26 on transglycosylation and its relationship to the neighboring acidic residue Asp20 by neutron crystallography, the perdeuterated recombinant proteins of the WT and Thr26His mutant T4L were prepared for crystallization in this study. The perdeuterated forms were produced in  cells cultured in deuterated rich media. After purification, macroseeding was performed to grow large crystals by transferring individual crystals to hanging drops. A crystal of Thr26His mutant T4L with a volume of 0.1 mm
cells cultured in deuterated rich media. After purification, macroseeding was performed to grow large crystals by transferring individual crystals to hanging drops. A crystal of Thr26His mutant T4L with a volume of 0.1 mm was grown after one month. Preliminary neutron-diffraction experiment at the research reactor FRM-II (Munich, Germany) at 100 K gave diffraction spots beyond 2.5
was grown after one month. Preliminary neutron-diffraction experiment at the research reactor FRM-II (Munich, Germany) at 100 K gave diffraction spots beyond 2.5  resolution for 1.5 hour exposure.
resolution for 1.5 hour exposure.
Hirano, Yu; Kurihara, Kazuo; Kusaka, Katsuhiro*; Miki, Kunio*; Tamada, Taro
no journal, ,
Most of electron transfer proteins possess prosthetic groups, such as heme, flavin and iron-sulfur cluster, and electron transfer reaction is mediated by hydrogen atoms or valence shell electrons of the prosthetic groups. Therefore, structural information including hydrogen atoms is required for understanding their electron transfer mechanisms. We have performed high-resolution neutron structure analyses with 2 electron transfer proteins of high-potential iron-sulfur protein (HiPIP) and NADH-cytochrome 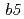 reductase (b5R). Diffraction experiments were performed at BL03 (iBIX) beamline of J-PARC using large crystals of HiPIP and b5R. Diffraction spots were observed up to 1.17
reductase (b5R). Diffraction experiments were performed at BL03 (iBIX) beamline of J-PARC using large crystals of HiPIP and b5R. Diffraction spots were observed up to 1.17  and 1.37
and 1.37  resolutions for HiPIP and b5R, respectively.
resolutions for HiPIP and b5R, respectively.
Tomoyori, Katsuaki; Kurihara, Kazuo; Tamada, Taro; Kuroki, Ryota
no journal, ,
Especially, large proteins such as membrane proteins or large protein complex are among the most important subjects of studies in structural biology. It will be greatly advance the fields of life science to elucidate the protein function from the structural information of hydrogen atoms and hydration waters obtained by neutron protein crystallography. Our group proposes the diffractometer which can cover such crystals with large unit cell volume (target lattice length: 250  ). We chose a decoupled liquid hydrogen moderator as an appropriate source for this high resolution diffractometer from both view points of flux and pulse width. Firstly, the problem of overlapped Bragg peaks becomes significant for high order reflections. Secondly, the large unit cell volume causes weak signals from protein crystal. The angular resolution will dominates at low angles, but time-resolution cannot be neglected with increasing the scattering angle. Consequently, the peak separation of Bragg reflection by the long flight-path will become more efficient to get over the first problem. To overcome second problem, it should be noted that the appropriate geometrical alignment of neutron supermirror guide, the selection of critical angle, and the elimination of fast neutron and
). We chose a decoupled liquid hydrogen moderator as an appropriate source for this high resolution diffractometer from both view points of flux and pulse width. Firstly, the problem of overlapped Bragg peaks becomes significant for high order reflections. Secondly, the large unit cell volume causes weak signals from protein crystal. The angular resolution will dominates at low angles, but time-resolution cannot be neglected with increasing the scattering angle. Consequently, the peak separation of Bragg reflection by the long flight-path will become more efficient to get over the first problem. To overcome second problem, it should be noted that the appropriate geometrical alignment of neutron supermirror guide, the selection of critical angle, and the elimination of fast neutron and  rays are required. We concisely estimated the instrumental resolution and intensity at sample position, and explain the result of the maximum resolvable lattice constant and measurement time.
rays are required. We concisely estimated the instrumental resolution and intensity at sample position, and explain the result of the maximum resolvable lattice constant and measurement time.
Matsumoto, Atsushi; Takagi, Junichi*; Iwasaki, Kenji*
no journal, ,
Shimizu, Rumi; Hiromoto, Takeshi; Adachi, Motoyasu; Kuroki, Ryota; Kataoka, Misumi*; Ishikawa, Kazuhiko*
no journal, ,
For neutron crystal structure analysis of proteins, it is necessary to prepare large crystals in volume (several mm ) compared to that required for X-ray crystal structure analysis. To prepare the large volume crystal, we inevitably need much amount of purified protein. As a development in preparation of samples for neutron crystal structure analysis of proteins, we have tried to develop expression system for many kinds of protein using Eschericha coli, Brevibacillus, Pichia pastoris, cultivation cell and so on. Recently, we have succeeded in high level expression of cellulose (EGPf) derived from Archaea
) compared to that required for X-ray crystal structure analysis. To prepare the large volume crystal, we inevitably need much amount of purified protein. As a development in preparation of samples for neutron crystal structure analysis of proteins, we have tried to develop expression system for many kinds of protein using Eschericha coli, Brevibacillus, Pichia pastoris, cultivation cell and so on. Recently, we have succeeded in high level expression of cellulose (EGPf) derived from Archaea 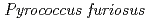 using Brevibacillus system.
using Brevibacillus system.
Adachi, Motoyasu; Maekawa, Keiko*; Matsuzawa, Yumiko*; Saito, Yoshiro*; Kuroki, Ryota
no journal, ,
CYP2C9 belongs to cytochrome P450 and play an important role as human drug metabolizing enzyme. In this study, to elucidate molecular mechanism in drug metabolizing reaction by wild-type CYP2C9 and its SNP mutant *30, X-ray structures of their CYP2C9 molecules complexed with the drug were determined. CYP2C9 was truncated at N-terminal, expressed in E. coli, and homogeneously purified for crystallization. CYP2C9 Losartan complex was crystallized by co-crystallization. As a result of experiments using synchrotron radiation, diffraction data were obtained at 2.1 resolution. The electron density maps of bound Losartan were observed in the both complexes.
resolution. The electron density maps of bound Losartan were observed in the both complexes.